Abstract
The rapid naturalization of Bombus terrestris across the Nemuro Peninsula has led to a decline in two closely related native Japanese species, namely Bombus hypocrita sapporensis and Bombus cryptarum florilegus, both belonging to the common subgenus Bombus. Although it is widely believed that cross-mating of native and non-native species is influenced by the common male sex pheromone in this region, no study has been conducted to substantiate this claim. Thus, we investigated the cross-activities of male sex pheromones between native and non-native bumblebees, as well as the frequencies of cross-mating, using chemical and DNA assays. Our gas chromatography–electroantennographic detector analyses and behavioral tests revealed the presence of sex pheromonal cross-activities between B. terrestris and the two Japanese bumblebees species. Furthermore, DNA analyses revealed the occurrence of cross-mating between native and non-native species in the Nemuro Peninsula. Overall, these results indicate the immediate need for conservation measures to safeguard Japanese bumblebee populations in the Nemuro Peninsula.
Similar content being viewed by others
Introduction
Since its designation as an alien species in Japan in 2006, the imported bumblebee Bombus terrestris L. has posed significant challenges. Although B. terrestris serves as an important greenhouse pollinator, escaped queens and males have established feral populations in Hokkaido, Japan. This naturalization process has led to competition between B. terrestris and native Japanese bumblebee species, the disruption of symbiotic relationships between native plants and bumblebees, and the introduction of new pests and pathogens1,2,3,4,5,6,7,8.
Under laboratory conditions, hybridization between B. terrestris and closely related Japanese bumblebee species, such as B. hypocrita hypocrita, B. h. sapporensis, and B. ignitus, occurs readily9. However, no viable hybrids are produced when native queens mate with B. terrestris males, as the laid eggs cease embryonic development10,11. Nevertheless, cross-mating between these species has detrimental effects on the reproduction of native bumblebees, as Bombus queens typically mate only once or twice12. Furthermore, previous DNA analysis revealed the presence of B. terrestris spermatozoa in the spermathecae of wild queens from B. h. hypocrita, B. h. sapporensis, and B. ignitus, indicating that cross-mating between B. terrestris males mate and native queens occurs in the field11. Our previous study also suggested that cross-mating is facilitated by the similarities in male sex pheromone production in the labial gland (LG)13.
The Nemuro Peninsula, located in eastern Hokkaido, represents a valuable habitat for native bumblebees, with 10 out of 15 Japanese species, including the rare bumblebee species, B. cryptarum florilegus, thriving in this region14. Indeed, B. c. florilegus exhibits a restricted distribution, occurring only in the Nemuro and Notsuke Peninsulas in Japan, and is listed as a near-threatened species in the Japan Red List14. Given that B. terrestris has naturalized in this region, the populations of B. h. sapporensis and B. c. florilegus have been steadily declining15. Although it is widely believed that cross-mating between native and non-native species is influenced by the common male sex pheromone in the region, there is no empirical evidence to support this hypothesis. Therefore, using chemical and DNA assays, we investigated the frequencies of cross-mating and the cross-activities of male sex pheromones between native and non-native bumblebees.
Results
Previous gas chromatography–electroantennographic detector (GC-EAD) analyses confirmed the presence of ethyl dodecanoate, 2,3-dihydrofarnesal, and 2,3-dihydrofarnesol emitted by the male LG in B. terrestris16. Additionally, ethyl dodecanoate has been detected in the male LG of B. h. sapporensis13. Our GC-EAD analyses confirmed that the male LG of B. c. florilegus emits ethyl dodecanoate. In addition, ethyl dodecanoate and 2,3-dihydrofarnesol elicited clear electrophysiological antennal responses in B. terrestris virgin queens (Fig. 1Aa). However, only ethyl dodecanoate evoked clear electrophysiological antennal responses in B. h. sapporensis and B. c. florilegus virgin queens (Fig. 1Ab,Ac,Ba,Bb,Bc,Ca,Cb,Cc).
Simultaneous gas chromatography–flame ionization detector and electroantennographic detector recordings from (a) Bombus terrestris, (b) B. hypocrita sapporensis, and (c) B. cryptarum florilegus virgin queens using volatiles collected from the male labial gland (LG) of (A) B. terrestris, (B) B. h. sapporensis, and (C) B. c. florilegus. Identified compounds: (1) ethyl dodecanoate; (2) 2,3-dihydrofarnesal; and (3) 2,3-dihydrofarnesol.
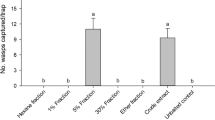
Binomial test analysis of behavioral tests conducted using a Y-tube olfactometer revealed that B. terrestris queens (n = 20) showed a significant (P < 0.05) preference for BtL (P = 0.000067), BhsL (P = 0.023103), BcfL (P = 0.044357), ED (P = 0.00109), DF (P = 0.000067), and EFM (P = 0.000067) over pentane (Fig. 2). Similarly, based on binomial test analysis, B. h. sapporensis queens exhibited a significant (P < 0.05) preference for BtL (P = 0.041656, n = 15), BhsL (P = 0.000214, n = 15), BcfL (P = 0.000366, n = 14), ED (P = 0.001221, n = 12), and EFM (P = 0.034912, n = 13) over pentane (Fig. 3). Likewise, binomial test analysis revealed that B. c. florilegus queens (n = 10) showed a significant (P < 0.05) preference for BtL (P = 0.017578), BhsL (P = 0.006836), BcfL (P = 0.006836), ED (P = 0.006836), and EFM (P = 0.043945) over pentane (Fig. 4). However, neither B. h. sapporensis (n = 11) nor B. c. florilegus queens (n = 10) exhibited attraction toward or repulsion from DF compared with pentane (binomial test, P = 0.225586 and P = 0.246094, respectively) (Figs. 3 and 4). Furthermore, B. terrestris (n = 20), B. h. sapporensis (n = 15), and B. c. florilegus (n = 10) queens exhibited no attraction toward or repulsion from pentane compared with an empty control (binomial test, P = 0.160179, P = 0.196381, and P = 0.205078, respectively) (Figs. 2, 3, and 4).
Attractiveness of labial gland (LG) extract scents from Bombus terrestris (BtL), B. h. sapporensis (BhsL), and B. c. florilegus (BcfL), as well as ethyl dodecanoate (ED), 2,3-dihydrofarnesol (DF), and a mixture of ethyl dodecanoate and 2,3-dihydrofarnesol (EFM), against the solvent pentane (Pe) and an empty control (E) in Bombus terrestris virgin queens. The y-axis indicates the number of individuals choosing each scent within 5 min. Asterisks indicate statistical significance (P < 0.05) determined through binomial tests.
Attractiveness of scents from labial gland (LG) extracts from Bombus terrestris (BtL), B. h. sapporensis (BhsL), and B. c. florilegus (BcfL), as well as ethyl dodecanoate (ED), 2,3-dihydrofarnesol (DF), and a mixture of ethyl dodecanoate and 2,3-dihydrofarnesol (EFM), against the solvent pentane (Pe) and an empty control (E) in B. h. sapporensis virgin queens. The y-axis indicates the number of individuals choosing either scent within 5 min. Asterisks indicate statistical significance (P < 0.05) determined through binomial tests.
Attractiveness of scents from labial gland (LG) extracts from Bombus terrestris (BtL), B. h. sapporensis (BhsL), and B. c. florilegus (BcfL), as well as ethyl dodecanoate (ED), 2,3-dihydrofarnesol (DF), and a mixture of ethyl dodecanoate and 2,3-dihydrofarnesol (EFM), against the solvent pentane (Pe) and an empty control (E) in B. c. florilegus virgin queens. The y-axis indicates the number of individuals choosing either scent within 5 min. Asterisks indicate statistical significance (P < 0.05) determined through binomial tests.
Mitochondrial DNA cytochrome c oxidase subunit I (COXI) gene sequences extracted from the sperm-derived DNA were found to have a 100% match with the sequences registered for each of the three species in the DNA Data Bank of Japan (LC695022, LC695025, and LC695021). We successfully decoded the ITS2 region of the three bumblebee species, which exhibited species-specific mutations. The ITS2 sequences of these species were registered in the DNA Data Bank of Japan (LC769002–LC769004). Notably, the ITS2 sequences obtained from sperm-derived DNA in the spermathecae of several queens showed a different species identification. The identification of cross-mating queens based on the ITS2 region was in agreement with the results of mitochondrial DNA analysis.
The results obtained from species-specific polymerase chain reaction (PCR) and sequence analysis were consistent, revealing that 9.9% of B. c. florilegus queens in the Nemuro Peninsula were inseminated by invading B. terrestris males. Additionally, 4.4% of B. h. sapporensis queens stored sperm from B. terrestris males in their spermathecae. Conversely, we observed no interbreeding between B. terrestris queens and the males of native species. Through dissection, we confirmed the absence of male sperm in the spermathecae of some queens of the three species. The frequency of unmated queens was found to be was 13.5%, 2.8%, and 2.8% for B. c. florilegus, B. h. sapporensis, and B. terrestris respectively (Table 1).
We observed overwrapped double peaks in the chromatograms of the mitochondrial DNA COXI gene sequences only in the DNA samples extracted from the spermathecae of B. h. sapporensis queens. Diagnostic PCR analyses of these samples revealed that 1.6% of the spermathecae in B. h. sapporensis queens contained DNA from both B. h. sapporensis and B. terrestris (Table 1 and Fig. 5).
(a) Electrophoretic gel image of PCR products using common (1) and specific primers (2–5) for Bombus cryptarum florilegus and B. terrestris. The gel shows the DNA marker (M), DNA samples from the leg (1 and 2) and spermatheca (3 and 4) of B. c.f. queens, and DNA samples from the legs of B. terrestris queens (5) (spermatheca inseminated by conspecific male 3, and spermatheca inseminated by allospecific B. terrestris male 4). (b) Electrophoretic gel image of PCR products using common (6) and specific primers (7–11) for B. hypocrita sapporensis and B. terrestris. The gel shows the DNA marker (M), DNA samples from the leg (6 and 7) and spermatheca (7–10) of B. h. s. queens, and DNA samples from the legs of B. terrestris queens (11) (spermatheca inseminated by conspecific male 8, spermatheca inseminated by conspecific and allospecific B. terrestris males 9, and spermatheca inseminated by allospecific B. terrestris male 10). (c) Electrophoretic gel image of PCR products using common (12) and specific primers (13 and 14) for B. terrestris. The gel shows the DNA marker (M), as well as DNA samples from the leg (12 and 13) and spermatheca (14) of B. terrestris queens.
Discussion
Our GC-EAD analyses and behavioral tests provided evidence of cross-activities in sex pheromones between B. terrestris and two native Japanese bumblebee species, B. h. sapporensis and B. c. florilegus. The attractiveness of ethyl dodecanoate and 2,3-dihydrofarnesol to virgin B. terrestris queens, and only ethyl dodecanoate to B. terrestris, B. h. sapporensis, and B. c. florilegus queens, was demonstrated. Scent-marking with odorants produced by the cephalic part of the LG is a common behavior among male bumblebees to attract conspecific queens17,18. Each bumblebee species has its own species-specific blend of scent-marking components19. Although it is believed that scent-marker pheromones play a role in reproductive isolation, and that the common presence of ethyl dodecanoate and 2,3-dihydrofarnesol in the LG leads to cross-mating between native and non-native species13, to the best of our knowledge, the present study is the first to demonstrate their effects on queens’ behavior. Our findings indicate that escaped B. terrestris males mate with virgin queens of native species in Nemuro, Hokkaido, Japan, suggesting that the similarity in male sex pheromones between native and non-native bumblebees is a key factor in cross-mating the field. Although a common sex pheromone suggests the potential for cross-mating between B. h. sapporensis and B. c. florilegus, no cross-mating between native bumblebees was observed in this study. We speculate that due to differences in their reproductive seasons and strategies, the virgin queens and males of the two native bumblebee species rarely encounter each other in the field.
DNA analyses confirmed the occurrence of cross-mating between native and non-native species in the Nemuro Peninsula. This may be attributed to DNA sample contamination or the possibility of queens being inseminated by B. h. sapporensis and B. terrestris males. Although temperate B. c. florilegus queens are monandrous12, B. h. sapporensis and B. terrestris exhibit both monandrous and polyandrous behaviors in Japan20. It has been reported that 34.0% of B. h. sapporensis queens are polyandrous. Interestingly, native bumblebee males did not mate with B. terrestris queens, potentially due to the territorial dominance of B. terrestris males during premating behavior involving scent-marking17,18. This reproductive interference likely leads to a decline in native bumblebee populations because native queens that mate with B. terrestris do not produce viable offspring owing to the arrested embryonic development of laid eggs10,11.
The decline of native bumblebee populations in Japan, particularly those of B. h. sapporensis and B. c. florilegus, has been increasing due to the rapid naturalization of B. terrestris, resulting in habitat degradation and the fragmentation of B. c. florilegus populations15. Indeed, a recent study highlighted the fragmented population and reduced genetic diversity of B. c. florilegus in the Nemuro and Notsuke Peninsulas14. These findings, along with our results, underscore the need for immediate conservation measures to protect native bumblebees in the Nemuro Peninsula. Furthermore, future studies should consider control methods for feral B. terrestris.
Methods
Bumblebees
Virgin queens and males of B. h. sapporensis and B. c. florilegus were obtained from laboratory-reared colonies, unless otherwise specified. These colonies were established using mated queens collected from the Nemuro Peninsula, Hokkaido Island, Japan, between June 2011 and 2012 (Fig. 6). Additionally, a commercial colony of B. terrestris L. was purchased from Agrisect Inc. (Inashiki, Ibaraki, Japan). All colonies were reared in an air-conditioned room at 28 °C under constant darkness with a diet of pollen and a 55% sugar solution. Electrophysiological analyses and behavioral tests were conducted using 7-day-old males and 7–11-day-old new queens.
Distribution map of Bombus species sampling locations in Japan (a). Bombus cryptarum florilegus (bcf) is distributed only in the Nemuro and Notsuke Peninsulas (c), marked by dashed lines. Bombus hypocrita sapporensis (bhs) is distributed across Hokkaido Island (b), indicated by white and gray shading. Bombus terrestris (bt) is widely present and becoming naturalized across Hokkaido Island, indicated by gray shading. The dashed area represents the overlapping distributions of three Bombus species. Map created using, Vactor trial release (Geospatial Information Authority of Japan), https://maps.gsi.go.jp/vector/.
Between 2009 and 2019, 641 queens of B. c. florilegus, B. h. sapporensis, and B. terrestris were collected from the Nemuro Peninsula, Hokkaido Island, Japan (Fig. 6). The collection details are provided in Table 1. For DNA analysis, queens were preserved in 99% ethanol and stored at − 20 °C.
Sample preparation for chemical analysis
Sample preparation for the analysis of male sex pheromones followed the method described in our previous study13. Male bumblebees were frozen at − 40 °C before chemical analysis and dissection to obtain LGs from the head. The LGs were placed in 4-mL vials, which were sealed with aluminum foil. Volatile components were extracted from the crushed LGs using solid-phase microextraction (SPME) headspace sampling for 10 min. The SPME device (SUPELCO; Sigma-Aldrich, St. Louis, Missouri, USA) included a fused-silica fiber coated with polydimethylsiloxane (100-µm thickness).
GC-EAD analysis
The recording technique for antennal responses followed our previous study21. The GC-EAD system (TAIYO Co., Tsukuba, Japan) was used to investigate the antennal responses of B. terrestris, B. h. sapporensis, and B. c. florilegus virgin queens to extracts from the male LGs of conspecific and allospecific individuals. Volatiles were detected and separated using a DB-5MS column equipped with a GC-7890A system (Agilent Technologies, USA). The column temperature was initially set at 120 °C and then increased to 250 °C at a rate of 10 °C/min. Helium flow (6.5 mL/min) was used in a splitless injector port (250 °C), and a flame ionization detector port (300 °C) was used with a combination of hydrogen gas and air flow (30 mL/min and 400 mL/min, respectively).
Silver electrodes filled with conductive gel (Aquasonic; Parker Laboratories, USA) were connected to an amplifier. Antennae were extracted from live new queens using tweezers and positioned between the silver electrodes. Each analysis was repeated five times. Compounds that elicited responses in the antennae were considered EAD-active compounds.
To identify the EAD-active compounds, LG extracts were analyzed using gas chromatography–mass spectrometry following the same method used in GC-EAD analyses. Detected peaks were compared against the mass spectral database (Wiley 229), and presumed compounds were identified by comparing retention times and mass spectra with the respective chemical standards: 97% ethyl dodecanoate (Sigma-Aldrich) and 97% 2,3-dihydrofarnesol (TAIYO Co.). The identification 2,3-dihydrofarnesal was based on a comparison of the obtained mass spectra with those in the Wiley library and previously published data16.
Behavioral experiment
Bioassays of queen bumblebees, used to assess their response to conspecific or allospecific male LG extracts, followed methods described previously21,22. In the behavioral experiment, a Y-tube olfactometer comprising a single long arm (length, 35.0 cm; diameter, 3.5 cm) and two short arms (length, 15.0 cm; diameter, 3.5 cm) was used. Glass cylinders (length, 10.0 cm; diameter, 2.5 cm) containing the test substances were connected to the end of the shorter arms using silicone tubing. The test substances included 2 μL of 0.001% ethyl dodecanoate (ED), 2,3-dihydrofarnesol (DF), a mixture of ethyl dodecanoate and 2,3-dihydrofarnesol (EFM) dissolved in pentane, as well as LG extracts of B. terrestris (BtL), B. h. sapporensis (BhsL), B. c. florilegus (BcfL), and pentane alone. These substances were applied to a piece of filter paper in the glass cylinder. The LG extracts were obtained by immersing male LGs in 1 mL of pentane for 20 min. Both glass cylinders were connected to an air pump via silicone tubes of equal length, with air forced into each cylinder at a rate of 3 mL/min through a single inlet.
In total, 20, 16, and 10 virgin queens of B. terrestris, B. h. sapporensis, and B. c. florilegus, respectively, were included in the bioassays. Each queen was released into a plastic chamber (15 × 10 × 10 cm) connected to the long arm of the Y-tube. If a bee chose one of the shorter arms within 5 min, it was considered to have ‘‘chosen’’ the corresponding odorant. Bees that did not make a choice within this timeframe were excluded from the analysis. The experimental sequence consisted of choices between empty (E) vs. pentane, followed by BtL vs. pentane, BhsL vs. pentane, BcfL vs. pentane, ED vs. pentane, DF vs. pentane, and EFM vs. pentane. To prevent potential bias toward a specific arm of the Y-tube, the positions of the treatment (BtL, BhsL, BcfL, ED, DF, and EFM) and control (pentane) were changed after each run. The design of the Y-tube apparatus is shown in Supplementary Fig. 7.
Statistical analysis
Data obtained from the Y-tube experiment were analyzed using the binomial test. P values for multiple comparisons were corrected using Holm’s method.
DNA extraction from individuals and spermathecae
Genomic DNA from queens was extracted from the hind legs using the DNeasy Blood & Tissue Kit (QIAGEN). Sperm present in the spermathecae of queens was collected and processed as described previously23. Spermathecae were dissected in 1 × phosphate-buffered saline solution, and sperm clumps were separated from the membranes using insect pins. Genomic DNA from the male that mated with a queen was extracted from these sperm clumps using the DNeasy Blood & Tissue Kit (QIAGEN).
Mitochondrial DNA sequence analysis
Fragments of the mitochondrial COXI gene, commonly used as a DNA barcoding region, were amplified via PCR using the primer pair COIF (HCO2198_t1: 5ʹ-TGTAAAACGACGGCCAGTGGTCAACAAATCATAAAGATATTGG-3ʹ) and COIR (LCO1490_t1: 5ʹ-CAGGAAACAGCTATGACTAAACTTCAGGGTGACCAAAAAATCA-3ʹ)24. The PCR amplifications consisted of an initial denaturation step at 98 °C for 2 min, followed by 30 cycles of denaturation at 98 °C for 10 s, annealing at 50 °C for 30 s, extension at 72 °C for 1 min, and a final extension at 72 °C for 10 min. ExTaq DNA polymerase (Takara, Otsu, Japan) was used for amplifications in a thermal cycler (Takara). PCR products were purified using ExoSAP-IT (USB Corporation, Cleveland, OH). Cycle sequencing reactions were performed using both primers and BigDye Terminator v.3.1 (Applied Biosystems). After product purification, sequencing was performed using an ABI 3130xl sequencer (Applied Biosystems). The obtained sequences were aligned using the GENETYX program (GENETYX, Tokyo, Japan), and a BLAST search was conducted for homology analysis.
Determination by diagnostic PCR
Interspecific copulation between B. c. florilegus, B. h. sapporensis, and B. terrestris was confirmed through PCR and electrophoresis. Diagnostic PCR was conducted using bumblebee common and species-specific primers designed based on the COI gene sequences of B. c. florilegus, B. h. sapporensis, and B. terrestris, respectively25 (Table 2). The universal primer pair for DNA barcoding24 served as a positive control for DNA quality evaluation. The DNA used in the sequence analysis served as the template DNA. PCR was performed using the following cycle parameters: an initial denaturing step of 2 min at 95 °C, followed by 40 cycles of 15 s at 95 °C, 30 s at 50–58 °C, and 60 s at 72 °C. The final elongation step was extended to 10 min. HiDi DNA polymerase (myPOLS Biotec, Konstanz, Germany) was used in a thermal cycler (Takara). The PCR products were electrophoresed on 4% PrimeGel™ Agarose PCR-Sieve agarose gels (Takara) and visualized under ultraviolet light using Midorigreen Direct DNA Stain solution (NIPPON Genetics, Tokyo, Japan).
Nuclear DNA ITS2 region sequence analysis
To determine the presence of interspecific mating queens, we also sequenced the nuclear DNA ITS2 region. Randomly selected queens that were determined to have undergone interspecific mating based on diagnostic PCR for mitochondrial DNA, along with 20 queens per species determined to have undergone intraspecific mating, were analyzed. The ITS2 region was sequenced using two primers and experimental conditions developed previously26. The ITS2 sequence lengths of B. h. sapporensis, B. c. florilegus, and B. terrestris were approximately 2000 bp. The PCR fragments were ligated into a pUC19 vector and transformed into Escherichia coli JM109 competent cells (Takara, Otsu, Japan). In total, 360 recombinant colonies were picked and individually suspended in 20 μL of TE buffer. The inserts were amplified through PCR using M13-RV and M13–47 primers for the multicloning site of pUC19 (Takara, Otsu, Japan) following the manufacturer’s instructions. Three internal primers common to these bumblebees were designed for sequencing (BombusITS2_588: 5ʹ-GCAGGTTTTCGATGAGCACG-3ʹ; BombusITS2_1103: 5ʹ-ACGTTCGTCGGAAATCGTAC-3ʹ; BombusITS2_1474: 5ʹ-GTTGGTCATCCCATGCCTTT-3ʹ). The sequencing analysis method was the same as that used for sequencing the mitochondrial DNA COXI gene, as described above.
Data availability
Mitochondrial DNA sequences of three Bombus species are available in the DNA Data Bank of Japan: Bombus cryptarum: http://getentry.ddbj.nig.ac.jp/getentry/na/LC695021/?filetype=html; Bombus hypocrita: http://getentry.ddbj.nig.ac.jp/getentry/na/LC695025/?filetype=html; Bombus terrestris: http://getentry.ddbj.nig.ac.jp/getentry/na/LC695022/?filetype=html. Additionally, nuclear DNA ITS2 sequences of three Bombus species are available in the DNA Data Bank of Japan: Bombus cryptarum: http://getentry.ddbj.nig.ac.jp/getentry/na/LC769002/?filetype=html; Bombus hypocrita: http://getentry.ddbj.nig.ac.jp/getentry/na/LC769003/?filetype=html; Bombus terrestris: http://getentry.ddbj.nig.ac.jp/getentry/na/LC769004/?filetype=html.
References
Goka, K., Okabe, K., Yoneda, M. & Niwa, S. Bumblebee commercialization will cause worldwide migration of parasitic mites. Mol. Ecol. 10, 2095–2099 (2001).
Matsumura, C., Yokoyama, J. & Washitani, I. Invasion status and potential ecological impacts of an invasive alien bumblebee, Bombus terrestris L. (Hymenoptera: Apidae) naturalized in southern Hokkaido, Japan. Glob. Environ. Res. 8, 51–66 (2004).
Inari, N., Nagamitsu, T., Kenta, T., Goka, K. & Hiura, T. Spatial and temporal pattern of introduced Bombus terrestris abundance in Hokkaido, Japan and its potential impact on native bumblebees. Popul. Ecol. 47, 77–82 (2005).
Goka, K., Okabe, K. & Yoneda, M. Worldwide migration of parasitic mites as a result of bumblebee commercialization. Popul. Ecol. 48, 285–291 (2006).
Kenta, T., Inari, N., Nagamitsu, T., Goka, K. & Hiura, T. Commercialized European bumblebee can cause pollination disturbance: An experiment on seven native plant species in Japan. Biol. Conserv. 134, 298–309 (2007).
Dohzono, I. K., Kunitake, K. Y., Yokoyama, J. & Goka, K. Alien bumble bee affects native plant reproduction through interactions with native bumble bees. Ecology 89, 3082–3092 (2008).
Inoue, M. N., Yokoyama, J. & Washitani, I. Displacement of Japanese native bumblebees by the recently introduced Bombus terrestris (L.) (Hymenoptera: Apidae). J. Insect Conserv. 12, 135–146 (2008).
Ishii, H. S., Kadoya, T., Kikuchi, R., Suda, S. & Washitani, I. Habitat and flower resource partitioning by an exotic and three native bumblebees in central Hokkaido, Japan. Biol. Conserv. 141, 2597–2607 (2008).
Ono, M. Ecological implication of introduced Bombus terrestris, and significance of domestication of Japanese native bumblebees (Bombus spp.). In Proc. International Workshop on Biological Invasions of Ecosystem by Pests and Beneficial Organisms. NIAES, Ministry of Agriculture, Forestry and Fisheries, Japan, Tsukuba, Japan. 244–252 (1997).
Kanbe, Y., Okada, I., Yoneda, M., Goka, K. & Tsuchida, K. Interspecific mating of the introduced bumblebee Bombus terrestris and the native Japanese bumblebee Bombus hypocrita sapporoensis results in inviable hybrids. Naturwissenschaften 95, 1003–1008 (2008).
Kondo, I. N. et al. Reproductive disturbance of Japanese bumblebees by the introduced European bumblebee Bombus terrestris. Naturwissenschaften 96, 467–475 (2009).
Takahashi, J., Itoh, M., Shimizu, I. & Ono, M. Male parentage and queen mating frequency in the bumblebee Bombus ignitus (Hymenoptera: Bombinae). Ecol. Res. 23, 937–942 (2008).
Kubo, R. & Ono, M. Comparative analysis of volatile components from labial glands of male Japanese bumblebees (Bombus spp.). Entomol. Sci. 13, 167–173 (2010).
Takeuchi, T. et al. Low mitochondrial DNA variation in the endangered bumble bee Bombus cryptarum florilegus. J. Apic. Res. 58, 591–596 (2019).
Takahashi, J. et al. Bumblebee fauna of the Nemuro Peninsula, Hokkaido, Japan: With special reference to the effect of the invasive Bombus terrestris on the rare B. florilegus. Jpn. J. Conserv. Ecol. 15, 101–110 (2010) (in Japanese).
Luxova, A., Urbanova, K., Valterova, I., Terzo, M. & Borg-Karlson, A. K. Absolute configuration of chiral terpenes in marking pheromones of bumblebees and cuckoo bumblebees. Chirality 16, 228–233 (2004).
Kullenberg, B., Bergström, G., Bringer, B., Carlberg, B. & Cederberg, B. Observations on scent marking by Bombus Latr. and Psithynus Lep. Males (Hymeno, Apidae) and localization of site of production of the secretion. Zoon Suppl. 1, 23–29 (1973).
Bergman, P. & Bergström, G. Scent marking, scent origin, and species specificity in male premating behavior of two Scandinavian bumblebees. J. Chem. Ecol. 23, 1235–1251 (1997).
Urbanova, K., Halik, J., Hovorka, O., Kindl, J. & Valterova, I. Marking pheromones of the cuckoo bumblebee males (Hymenoptera, Apoidea, Bombus Latreille): Compositions of labial-gland secretions of six species found in the Czech Republic. Biochem. Syst. Ecol. 32, 1025–1045 (2004).
Tsuchida, K., Yamaguchi, Y., Kanbe, Y. & Goka, K. Reproductive interference in an introduced bumblebee: Polyandry may mitigate negative reproductive impact. Insects. https://doi.org/10.3390/insects10020059 (2019).
Kubo, R., Harano, K. & Ono, M. Male scent-marking pheromone of Bombus ardens ardens (Hymenoptera; Apidae) attracts both conspecific queens and males. Sci. Nat. https://doi.org/10.1007/s00114-017-1493-1 (2017).
Brodmann, J. et al. Orchid mimics honey bee alarm pheromone in order to attract hornets for pollination. Curr. Biol. 19, 1368–1372 (2009).
Duvoisin, N., Bear, B. & Schmid-Hempel, P. Sperm transfer and male competition in a bumblebee. Anim. Behav. 58, 743–749 (1999).
Stahlhut, J. K. et al. DNA barcoding reveals diversity of Hymenoptera and the dominance of parasitoids in a sub-arctic environment. BMC Ecol. 13, 2 (2013).
Ueda, R. et al. Development of multiplex PCR primer sets for the identification of bumblebee species by modified DNA polymerase. DNA Polymorph. 31(1), 25–31 (2023) (in Japanese).
Oh, K. H. et al. ITS2 Ribosomal DNA sequence variation of the bumblebee, Bombus ardens (Hymenoptera: Apidae). Genes Genom. 31(4), 293–303 (2009).
Author information
Authors and Affiliations
Contributions
R.K., Y.A. and J.T. wrote the main manuscript text and H.O. and M.O. prepared all figures and supplement data. R.K. and M.O. did the chemical analysis. Y.A., E.F., H.O., and J.T. performed DNA analysis. All authors performed field research and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kubo, R., Asanuma, Y., Fujimoto, E. et al. Cross-mating between the alien bumblebee Bombus terrestris and two native Japanese bumblebees, B. hypocrita sapporensis and B. cryptarum florilegus, in the Nemuro Peninsula, Japan. Sci Rep 13, 11506 (2023). https://doi.org/10.1038/s41598-023-38631-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-38631-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.