Abstract
Expansion of wild and managed allochthonous species leads to potential negative consequences for the endemic wildlife, such as resource competition, pathogens spread, hybridization and native species replacements. On Capraia Island, the last sighting of Bombus terrestris terrestris dates back to 1917. All subsequent surveys carried out on the island only reported the presence of B. xanthopus and B. pascuorum melleofacies with B. t. terrestris apparently no longer existing in the area. In 2021 B. t. terrestris was again detected on the island raising two main hypotheses: (i) B. t. terrestris has always been present with a low population density, such as not to be detected in previous investigations, or (ii) its presence is the result of a more recent recolonization. The recolonization event may be promoted by either intentional or unintentional introduction or it may be the result of a natural migration from the mainland or surrounding islands. Hybridization between B. t. terrestris × B. xanthopus on Capraia Island has been also ascertained by the detection of hybrid queens, workers and males. These new finding provides insight on the distribution range of B. t. terrestris in the Tuscan Archipelago and raise concern on the conservation of the endemic B. xanthopus population.
Similar content being viewed by others
Introduction
Bombus terrestris (L. 1758) is a ubiquitous Palearctic species, occurring throughout Europe with a very high population density (Intoppa et al. 1995; Rasmont et al. 2008). In Europe seven morphological subspecies of B. terrestris are present: B. t. terrestris in continental Europe; B. t. audax Harris, 1780, in British Islands; B. t. calabricus Krüger, 1956, in South Italy and Sicily; B. t. canariensis Pérez, 1895, in Canary Islands; B. t. dalmatinus Dalla Torre, 1882, in farthest South-East France, North Italy, Balkanic Peninsulas and surrounding regions; B. t. lusitanicus Krüger, 1956, in South-West France, Iberian Peninsula, Balearic Islands and Madeira; B. t. sassaricus Tournier, 1890, in Sardinia (Rasmont et al. 2008). B. xanthopus Kriechbaumer, 1870, present in Corsica, Elba and Capraia Islands and considered as a subspecies of B. terrestris until 2015 (Rasmont et al., 2008), has been elevated to the status of endemic Corsican species due to its molecular and eco-chemical features (Lecocq et al. 2015; Rasmont et al., 2021). Nevertheless, the acceptance of the species assignment for the taxon xanthopus seems to be provisional, as the Poisson-tree-process (PTP) provides conflicting results, leaving the taxonomic nomenclature a still open issue (Williams, 2021). The phenology of B. t. terrestris is usually characterized by one queen generation and a flying period ranging from March to August (Pawlikowski et al. 2020). However, B. t. terrestris population in Mediterranean regions, characterized by mild climates, shows flexibility in phenology compared to the inland population. Such flexibility result in all year-round activity with two generations, one in autumn and the other in winter (Rasmont 1985; Ricciardelli d’Albore 1986; Rasmont and Adamski 1995; Rasmont et al. 2008), as also demonstrated in laboratory conditions (Beekman 1998; Beekman and Van Stratum 2000). Since the end of the 1980s, colonies of B. t. terrestris, B. t. sassaricus and B. t. dalmatinus have been reared and commercialized throughout Europe and Italy to increase the efficiency of pollination in greenhouse crops (i.e. tomatoes, strawberries, blueberries) (Velthuis and Van Doorn 2006; Chandler et al. 2019). Bumblebee commercialization has led to introduction of allochthonous subspecies with consequent alteration of the subspecies distribution range (Ings et al. 2005; Velthuis and Van Doorn 2006; Ghisbain et al., 2021). Colonies commercialization for crop pollination purpose and accidental importation in non-European countries also led to the establishment of feral colonies of B. terrestris in Tasmania (Semmens et al., 1993; Buttermore 1997), in Israel (Dafni and Shmida 1996) and in Chile (Ruz 2002), as well as in countries where it has now become naturalized, such as New Zealand (Goulson 2003) and Japan (Matsumura et al. 2004).
In Italy, four subspecies of B. terrestris exist: B. t. terrestris and B. t. dalmatinus Dalla Torre, 1882; B. t. calabricus Krüger, 1958; and B. t. sassaricus Tournier, 1890 (Rasmont et al. 2008). B. terrestris subspecies show differences in the colour pattern, behavioural traits (i.e. aggressiveness, colony dimensions, foraging performance) (Rasmont et al. 2008; Ings et al. 2010) and physiological features (De Jonghe 1986; Chittka et al. 2004). B. t. terrestris has a black coat with a yellow collar on the thorax, a yellow band on the abdomen (which has a white tip) and black legs (Ings et al. 2005). This colour pattern is very similar to that of other Bombus species present in Italy such as B. cryptarum (Fabricius, 1775) and B. lucorum (Linnaeus, 1761), the latter present only at altitudes over 500 m (Intoppa et al. 2009).
B. t. terrestris is widespread throughout mainland Tuscany at low altitudes and in the Archipelago (Intoppa et al. 1995; Rasmont and Quaranta 1997; Generani et al. 2001). The Tuscan Archipelago (Italy) includes seven islands, Gorgona, Capraia, Elba, Pianosa, Montecristo, Giglio and Giannutri extending along the north-south axis of Tyrrhenian Sea for a total of 295 km2 (www.islepark.it). Since the end of the 1970s, the presence of B. t. terrestris has been ascertained on all Tuscan Archipelago islands (Fanfani and Groppali 1979; Rasmont and Adamski 1995; Rasmont and Quaranta 1997; Generani et al. 1998, 2001; Cini et al. 2022) except Capraia. On Capraia Island, the last sighting of B. t. terrestris dates back to 1917 (Razzauti 1917). Since then, only B. xanthopus and B. pascuorum melleofacies were detected (Masi 1933; Rasmont and Adamski 1995; Rasmont and Quaranta 1997; Generani et al. 2001). Within a wider project focused to implement a monitoring network of bee species in the Tuscan Archipelago National Park and increase the knowledge on the ecology, biology and conservation status of these pollinators, the aim of this work was to assess the status of B. t. terrestris on Capraia Island and its potential impact on the endemic population of B. xanthopus.
Materials and methods
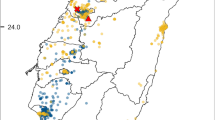
In summer-autumn 2021 and winter-spring 2022, a survey of bee (Apoidea: Anthophila) fauna in the Tuscan Archipelago National Park was performed (Ministero dell’Ambiente e della Tutela del Territorio e del Mare 2019). On Capraia Island (19 km2), a transect 250 m long and 2 m wide (45° 05′ 49.5″N; 9° 83′ 18.6″E) in Loc. Porto Vecchio (150 m a.s.l.) was defined according to guidelines for European pollinator monitoring scheme (Potts et al. 2020) (Fig. 1). The transect site was defined in agreement with the Tuscan Archipelago National Park to cover an area as representative as possible of the island’s environment (i.e. the presence of cultivated areas such as vineyards and Mediterranean shrub). The transect was walked once a month in May, July and September 2021 and in February and March 2022 for a total of 5 capture sessions performed, each lasting 1 h (i.e. from 12:00 to 13:00). During transect sampling, all present bees were captured by using an entomological net including specimens phenotypically attributable to B. t. terrestris. Each captured individual was placed in a 50-ml falcon tube containing chopped cork, euthanized by adding two drops of ethyl acetate and stored at −20°C until preparation for taxonomic identification. To each sample, an individual code containing capture information (i.e. date, site, and identification number of capture) was attributed. Taxonomic recognition was performed only on taped bumblebees individuals based on analysis of diagnostic morphological traits (Rasmont et al. 1986), hair and cuticle colours (Ruz 2002; Rasmont et al. 2008) using the identification key of Rasmont et al. (2021).
A Map of the Tuscan Archipelago, black lines indicate distances between Capraia and Elba, Corsica and Tuscany coast, B enlarged view of Capraia Island, the black square indicates the area in which bee fauna survey was performed and C detail of the survey area, the black line indicates the 250 m long transect on which bees were collected.
Results
During the survey 161 bees were collected of which 65 (40.4%) bumblebees, 20 in 2021 and 45 in 2022 (Table 1). B. t. terrestris was detected on Capraia in both 2021 and 2022: 1 ♀ (queen), 21.IX.2021, C.B. Boni leg., M. Quaranta det.; 2 ♂ 15.II.2022, F. Coppola leg., M. Quaranta det.; and 6 ♂ 1♀ (worker), 22.III.2022, F. Coppola, M. Quaranta det. for a total of 10 (15.4%) captured individuals. The collected specimens presented the following colour pattern: black facial and vertex hair; broad yellow collar, extending slightly below the tegulae, with isolated and sparse black hair at the margins of the collar; the rest of the thorax covered with black hair; legs with black cuticle covered with short black hair; black corbicula bristles; tergite 1 and tergite 3 black; tergite 2 yellow; tergite 4 and tergite 5 ivory white; and tergite 6 covered with short black hair (Fig. 2 and Fig. 3A). The 36.9% (n=24) of collected bumblebees during survey were assigned to B. xanthopus: 4 ♀ 1 ♀ (queen), 16.V.2021, F. Coppola leg., M. Quaranta det.; 3 ♀ 1 ♂, 18.VII.2021, C.B. Boni leg., M. Quaranta det.; 6 ♀ 1 ♂, 15.II.2022, C.B. Boni leg., M. Quaranta det.; and 8 ♀, 22.III.2022, F. Coppola leg., M. Quaranta det.. All the B. xanthopus specimens present black thorax with collar missing or restricted to a few yellow hairs; tergite 1 to tergite 3 entirely black or with restricted to a few yellow hairs; and tergite 4 to tergite 6 reddish and reddish cuticula with entirely reddish bristles (Fig. 3B).
A Queen of Bombus terrestris terrestris and its schematic colour pattern of B. terrestris terrestris: yellow collar and tergite 1, black legs cuticula and black corbicula bristles. B Worker of B. xanthopus, and its schematic colour pattern of B. xanthopus: black thorax, few yellow hairs on tergite 1, reddish legs cuticula and corbicula bristles.
On the Island, also hybrid specimens between B. xanthopus × B. t. terrestris were found in both years of survey for a total of 31 (47.7%) specimens: 6 ♀ 1 ♀ (queen) 1 ♂, 16.V.2021, F. Coppola leg., M. Quaranta det.; 2 ♂, 18.VII.2021, C.B. Boni leg., M. Quaranta det.; 5 ♀ 4 ♂, 15.II.2022, C.B. Boni leg., M. Quaranta det.; and 6 ♀ 1 ♀ (queen) 5 ♂ 22.III.2022, F. Coppola leg., M. Quaranta det.. Hybrids individuals presented different intermediate colour patterns between B. xanthopus and B. t. terrestris but always with reddish legs cuticula with entirely reddish cuticula bristles (Fig. 4).
Worker of B. t. terrestris, B. xanthopus and of B.t. xanthopus × B. t. terrestris showing different intermediate colour pattern collected on Capraia Island. A B. t. terrestris: yellow collar and tergite 1, black legs cuticula and black corbicula bristles; B B. xanthopus × B. t. terrestris: yellow collar and tergite 1, reddish legs cuticula and corbicula bristles; C B. xanthopus × B. t. terrestris with few yellow hairs on collar, yellow tergite 1, reddish legs cuticula and corbicula bristles; D B. xanthopus × B. t. terrestris: absence of collar, yellow tergite 1, reddish legs cuticula and corbicula bristles; E B. xanthopus: black thorax, few yellow hairs on tergite 1, reddish legs cuticula and corbicula bristles.
Discussion
Results obtained in this investigation provide new knowledge on B. t. terrestris distribution range within the Tuscan Archipelago and confirm the presence of this subspecies also on Capraia Island. Such finding occurs more than 100 years from Razzauti (1917) sighting. The detection of male, worker and queen individuals suggests the presence of at least one reproductive colony on the island. Two main hypotheses can be formulated for the presence of this bumblebee subspecies on Capraia: (i) B. t. terrestris has always been present on the Island with a low population density, such that it has gone unnoticed by other authors, or (ii) its presence is the result of a more recent colonization promoted by several factors such as intentional importation for pollination, unintentional transport by ferries and boats or by mass-migrating phenomena.
Although Razzauti (1917) does not provide any indication on the number of observed individuals or abundance, a low population density of B. t. terrestris could be hypothesized on Capraia since no other authors reported its presence in their checklist (Masi 1933; Rasmont and Adamski 1995; Rasmont and Quaranta 1997; Generani et al. 2001). Moreover, the absence of hybrids between B. xanthopus and B. t. terrestris on the island after Razzauti (1917) sighting of B. t. terrestris, allows to hypothesize that occurrence of this subspecies might have been an isolated and unique event.
Agriculture on Capraia Island is mainly based on olive growing, viticulture and fruit growing. Horticulture is only performed for family use and no greenhouses are present on the island (Felicioli 2021). Therefore, it is unlikely that B. t. terrestris has be imported on Capraia for pollination purposes. Conversely, the unintentional transport of B. t. terrestris individuals from Tuscan coasts or other Archipelago islands by ferries and boats that daily connect these areas cannot be excluded. Furthermore, a recent study performed in Northern Europe demonstrated that bumblebees are able to travel over long distances (up to 200 km), even crossing large water bodies via active flight aided by favourable wind currents (Fijen 2021). Individuals of the white-tailed species complex, such as B. terrestris, B. lucorum (Linnaeus, 1761) and B. ruderatus (Fabricius, 1775), were frequently observed flying at sea at about 50 km from the coast, either coming from the sea or flying towards the sea (Mikkola 1978; Fijen 2021). Capraia Island is 30 km from Elba, 40 km from Gorgona and 50 km from the coast of Tuscany, areas where B. t. terrestris is present. These distances are compatible with those travelled by bumblebees in Northern Europe (Fijen 2021). Therefore, migration from near islands or from the Tuscan coast is a solid hypothesis, and a phylogenetic analysis should be performed to better explain the origin of B. t. terrestris individuals present on Capraia Island. Little is known about the ecological and biological factors that determine bumblebee mass-migration. Data on migration timing and reasons on factors driving migration route and the choice of settle area are desirable. This bumblebee newly observed behavioural trait could strongly influence bumblebee distribution ranges and implies a renewal in the planning of conservation initiatives and in the management of endangered species. Further investigation on recolonization trends of bumblebee species or subspecies in areas of ancient presence is also need.
The distribution of B. xanthopus on Capraia Island is well known and documented since 1933 (Masi 1933), and its presence was also confirmed during this investigation. In this investigation, hybrids individuals between B. t. terrestris and B. xanthopus were recorded on Capraia Island for the first time, which indicates that warnings highlighted by Williams (2021) came true. Currently, no data on the abundance of B. xanthopus population on Capraia are available. However, considering the small dimension of the island, the occurrence of hybridization between B. t. terrestris and B. xanthopus, as already occurred on Elba Island (Kruger 1954; Rasmont and Adamski 1995; Rasmont and Quaranta 1997) and reported on the Tuscan coast (Quaranta and Felicioli 2012), is not surprisingly and raising concern on the conservation of the endemic B. xanthopus population. Impacts of the introduction of B. terrestris on native species have been already evaluated and documented worldwide (Inari et al. 2005; Winter et al. 2006; Dafni et al. 2010; Russo 2016). In Japan, imported B. terrestris, now considered naturalized, mate with a high frequency with the endemic B. hypocrita Pérez, 1905, producing inviable hybrid eggs and determining the potential decline of the native bumblebee (Kanbe et al. 2008; Kondo et al. 2009; Tsuchida et al. 2010, 2019). Investigation on interspecific hybridization between B. terrestris and B. ignitus shows that males of B. ignitus Smith, 1869, mate favourably with B. terrestris queens, leading to a potential genetic contamination of the endemic species (Yoon et al. 2009). In addition to genetic contamination, allochthonous B. terrestris could threaten the endemic population by competing for nest sites, spreading parasites and pathogens and inducing disturbances to the reproduction of the local flora (Hingston and McQuillan 1998; Matsumura et al. 2004; Yoneda et al. 2008; Nagamitsu et al. 2009; Dafni et al. 2010; Cilia et al. 2022).
Despite controversies over the taxonomy of B. xanthopus, the presence of hybrids individuals on the Island assumes relevance either they derive from an inter- or an intra-specific hybridisation. Fertility of hybrids B. t. terrestris × B. xanthopus, as well as B. t. terrestris × B. t. canariensis, has been yet demonstrated under laboratory condition (De Jonghe 1986; van den Eijnde and de Ruijter 2000), and for this reason, a potential spread of B. t. terrestris in Corsica and Canary Islands could cause threat to the endemic B. xanthopus and B. t. canariensis, respectively (Williams, 2021; Ghisbain et al., 2021). In this case, the risk of genetic contamination of the native subspecies could be potentially higher considering that mass-migration of B. t. terrestris in Mediterranean area could happen twice a year. Despite the general decline of pollinators worldwide, some species are recording expansion of their distribution areas, especially due to climate change and commercialisation for pollination purpose (Ghisbain et al., 2021), and in this context, B. t. terrestris could pose a severe threat for the genetic conservation of the endemic B. xanthopus.
Moreover, the presence of hybrids may increase the probability of the loss of the endemism throughout genetic contamination or the potential sterility of hybrid males.
The detection on Capraia Island of hybrids males indicates that hybrid queens in field condition can at least develop and lay haploid eggs. This evidence arises the urgency to establish the species status of the taxon xanthopus and then the fertility status of hybrids males since the presence of sterile males could act in a similar way of the male-sterile release technique.
In conclusion, results obtained in this investigation clearly indicate the presence of B. t. terrestris and B. t. terrestris × B. xanthopus hybrids on Capraia Island. These evidences raise several concerns about conserving endemic B. xanthopus populations that should prompt assessing the spread, the genetic origin and/or colonization pathways of B. t. terrestris on the island as well as the fertility status of hybrids. Furthermore, genetic analyses of hybrid individuals to establish the degree of hybridization and to exclude that intermediate phenotypes found on the Island may actually be part of the natural intraspecific variations of B. xanthopus population, are necessary. Resulting data will provide a useful tool for the development of a conservative action plan.
Data availability
All data are available from the corresponding author
References
Beekman M (1998) The bumblebee life cycle. Overwintering and colony growth of Bombus terrestris. PhD thesis, Universiteit van Amsterdam
Beekman M, Van Stratum P (2000) Does the diapause experience of bumblebee queens Bombus terrestris affect colony characteristics? Ecol Entomol 25(1):1–6
Buttermore RE (1997) Observations of successful Bombus terrestris (L.) (Hymenoptera: Apidae) colonies in southern Tasmania. Aust. J Entomol 36(3):251–254
Chandler D, Cooper E, Prince G (2019) Are there risks to wild European bumble bees from using commercial stocks of domesticated Bombus terrestris for crop pollination? J Apicul Res 58(5):665–681. https://doi.org/10.1080/00218839.2019.1637238
Chittka L, Ings TC, Raine NE (2004) Chance and adaptation in the evolution of island bumblebee behaviour. Popul Ecol 46(3):243–251
Cilia G, Flaminio S, Zavatta L, Ranalli R, Quaranta M, Bortolotti L, Nanetti A (2022) Occurrence of honey bee (Apis mellifera L.) pathogens in wild pollinators in Northern Italy. Front Cell Infect Microbiol 12:907489. https://doi.org/10.3389/fcimb.2022.907489
Cini A, Benetello F, Bonifacino M, Salvati V, Monterastelli E, Pasquali L, Sistri G, Dani FR, Dapporto L (2022) Simple and informative: applying a basic Anthophila monitoring scheme in a simplified insular ecosystem. Bull Insectology 75(1):83–95
Dafni A, Kevan P, Gross CL, Goka K (2010) Bombus terrestris, pollinator, invasive and pest: an assessment of problems associated with its widespread introductions for commercial purposes. Appl Entomol Zool 45(1):101–113
Dafni A, Shmida A (1996) The possible ecological implications of the invasion of Bombus terrestris (L.) (Apidae) at Mt. Carmel, Israel. In: Matheson A, Buchmann SL, O’Toole CH, Westrich P, Williams IH (eds) The conservation of bees. Academic, London, pp 183–200
De Jonghe R (1986) Crossing experiments with Bombus terrestris terrestris (Linneus, 1758) and Bombus terrestris xanthopus Kriechbaumer 1870 and some notes on diapause and nosemose (Hymenoptera: Apoidea). Phegea 14:19–23
Fanfani A, Groppali R (1979) La fauna di Montecristo - Arcipelago Toscano. (Studi sulla Riserva Naturale dell'Isola di Montecristo). Ist Entomol Univ Pavia 9:1–52
Felicioli A (2021) Relazione finale delle attività svolte nell’ambito del Progetto “Studio degli insetti impollinatori e sull’agricoltura tradizionale presso le isole di Capraia e Giglio”. Technical Report.
Fijen TP (2021) Mass-migrating bumblebees: an overlooked phenomenon with potential far-reaching implications for bumblebee conservation. J Appl Ecol 58(2):274–280
Generani M, Pagliano G, Scaramozzino P, Strumia E (1998) Nuovi Imenotteri dell’Isola di Montecristo. Frustula Entomol 21:75–83
Generani M, Pagliano G, Scaramozzino P, Strumia F (2001) Gli Imenotteri delle isole di Capraia, Giglio, Gorgona, Pianosa e Montecristo (Arcipelago Toscano). Frustula Entomol 24(37):51–74
Ghisbain G, Gérard M, Wood TJ, Hines HM, Michez D (2021) Expanding insect pollinators in the Anthropocene. Biol Rev 96(6):2755–2770
Goulson D (2003) Bumblebees: their behaviour and ecology. Oxford University Press, USA
Hingston AB, McQuillan PB (1998) Nectar robbing in Epacris impressa (Epacridaceae) by the recently introduced bumblebee Bombus terrestris (Apidae) in Tasmania. Vic Nat 115(4):116–119
Inari N, Nagamitsu T, Kenta T, Goka K, Hiura T (2005) Spatial and temporal pattern of introduced Bombus terrestris abundance in Hokkaido, Japan, and its potential impact on native bumblebees. Popul Ecol 47:77–82
Ings TC, Ings NL, Chittka L, Rasmont P (2010) A failed invasion? Commercially introduced pollinators in Southern France. Apidologie 41(1):1–13
Ings TC, Schikora J, Chittka L (2005) Bumblebees, humble pollinators or assiduous invaders? A population comparison of foraging performance in Bombus terrestris. Oecologia 144(3):508–516
Intoppa F, Piazza MG, Bolchi Serini G, Cornalba M (2009) I bombi. Guida al riconoscimento delle specie italiane. CRA - Unita di Ricerca di Apicoltura e Bachicoltura, Bologna
Intoppa F, Piazza MG, D'Albore GR (1995) Catalogo bibliografico delle specie di Bombidae (Hymenoptera Apoidea) segnalate per l'Italia. Supplement Apicult 10
Kanbe Y, Okada I, Yoneda M, Goka K, Tsuchida K (2008) Interspecific mating of the introduced bumblebee Bombus terrestris and the native Japanese bumblebee Bombus hypocrita sapporoensis results in inviable hybrids. Naturwissenschaften 95:1003–1008
Kondo NI, Yamanaka D, Kanbe Y, Kunitake YK, Yoneda M, Tsuchida K, Goka K (2009) Reproductive disturbance of Japanese bumblebees by the introduced European bumblebee Bombus terrestris. Naturwissenschaften 96:467–475
Krüger E (1954) Phänoanalytische Studien an einigen Arten der Untergattung Terrestribombus O. Vogt (Hymenoptera Bombidae). Teil Tijdschr Entomol 97:263–298
Lecocq T, Brasero N, De Meulemeester T, Michez D, Dellicour S, Lhomme P, De Jonghe R, Valterovà I, Urbanovà K, Rasmont P (2015) An integrative taxonomic approach to assess the status of Corsican bumblebees: implications for conservation. Anim Conserv 18(3):236–248
Masi L (1933) Raccolte entomologiche nell'isola di Capraia fatte da C. Mancini e E. Capra (1927-1931). III. Hymenoptera Aculeata, vol 11. Memorie della Società Entomologica italiana, Genova, pp 181–205
Matsumura C, Nakajima M, Yokoyama J, Washitani I (2004) High reproductive ability of an alien bumblebee invader, Bombus terrestris L., in the Hidaka region of southern Hokkaido, Japan. Japanese. J Conserv Ecol 9(1):93–101
Mikkola K (1978) Spring migrations of wasps and bumble bees on the southern coast of Finland (Hymenoptera, Vespidae and Apidae). Ann Zool Fenn 44:10–26
Ministero dell’Ambiente e della Tutela del Territorio e del Mare (2019) Direttiva agli enti parco nazionali e alle aree marine protette per l’indirizzo delle attività dirette alla conservazione della biodiversità. Prot. 0023838/UDCM of 12/10/2019
Nagamitsu T, Yamagishi H, Kenta T, Inari N, Kato E (2009) Competitive effects of the exotic Bombus terrestris on native bumble bees revealed by a field removal experiment. Popul Ecol 52(1):123–136
Pawlikowski T, Sparks TH, Olszewski P, Pawlikowski K, Rutkowski L, Jakubowski R (2020) Rising temperatures advance the main flight period of Bombus bumblebees in agricultural landscapes of the Central European Plain. Apidologie 51:652–663
Potts S, Dauber J, Hochkirch A, Oteman B, Roy D, Ahnre K, Biesmeijer K, Breeze T, Carvell C, Ferreira C, Fitzpatrick Ú, Isaac N, Kuussaari M, Ljubomirov T, Maes J, Ngo H, Pardo A, Polce C, Quaranta M, Settele J, Sorg M, Stefanescu C, Vujic A (2020) Proposal for an EU pollinator monitoring scheme, EUR 30416 EN, Publications Office of the European Union, Luxembourg, ISBN 978-92-76-23859-1, doi:10.2760/881843, JRC122225
Quaranta M, Felicioli A (2012) First report of Bombus terrestris xanthopus Kriechbaumer on the Italian peninsula (Hymenoptera: Apidae). Ann Soc Entomol Fr 48(3-4):343–346
Rasmont P (1985) Bombus terrestris (L.) (Hymenoptera, Apidae) dans le Massif des Maures (France, Var), une génération d’hiver. Bull Ann Soc R Belge Entomol 120:359–363
Rasmont P, Adamski A (1995) Les bourdons de la Corse (Hymenoptera, Apoidea, Bombinae). Notes Fauniques Gembloux 31:3–87
Rasmont P, Coppée A, Michez D, De Meulemeester T (2008) An overview of the Bombus terrestris (L. 1758) subspecies (Hymenoptera: Apidae). Ann Soc Entomol Fr 44(2):243–250
Rasmont P, Ghisbain G, Terzo M (2021) Bumblebees of Europe and neighbouring regions. Nap Editions
Rasmont P, Quaranta M (1997) I Bombi dell’Arcipelago Toscano. Boll Soc Entomol Ital 129:31–38
Rasmont P, Scholl A, De Jonghe R, Obrecht E, Adamski A (1986) Identité et variabilité des mâles de bourdons du genre Bombus Latreille sensu stricto en Europe occidentale et centrale (Hymenoptera, Apidae, Bombinae). Rev Suisse Zool 93(3):661–682
Razzauti A (1917) Contributi alla conoscenza faunistica delle isole toscane. I. Isola di Capraia. Atti Soc Tosc Sci Nat Pisa Mem 31:196–224
Ricciardelli d’Albore G (1986) Bombus Latr. e Psithyrus Lep. in Umbria. Redia 69:171–256
Russo L (2016) Positive and negative impacts of non-native bee species around the world. Insects 7(4):69. https://doi.org/10.3390/insects7040069
Ruz L (2002) Bee pollinators introduced to Chile: a review. In: Kevan P, Fonseca IVL (eds) Pollinating Bees - The Conservation Link Between Agriculture and Nature. Ministry of Environment, Brasília, pp 155–167
Semmens TD, Turner E, Buttermore R (1993) Bombus terrestris (L.) (Hymenoptera: Apidae) now established in Tasmania. J Aust Entomol Soc 32(4):346
Tsuchida K, Kondo NI, Inoue MN, Goka K (2010) Reproductive disturbance risks to indigenous Japanese bumblebees from introduced Bombus terrestris. Appl Entomol Zool 45(1):49–58
Tsuchida K, Yamaguchi A, Kanbe Y, Goka K (2019) Reproductive interference in an introduced bumblebee: polyandry may mitigate negative reproductive impact. Insects 10:59. https://doi.org/10.3390/insects10020059
van den Eijnde J, de Ruijter A (2000) Bumblebees from the Canary Islands: mating experiments with Bombus terrestris L. from the Netherlands. In Sommeijer MJ, Meeuwsen FJAJ (eds.) Proceedings of the Section Experimental and Applied Entomology of the Netherlands Entomological Society 11:159-161
Velthuis HH, Van Doorn A (2006) A century of advances in bumblebee domestication and the economic and environmental aspects of its commercialization for pollination. Apidologie 37(4):421–451
Williams PH (2021) Not just cryptic, but a barcode bush: PTP re-analysis of global data for the bumblebee subgenus Bombus s. str. supports additional species (Apidae, genus Bombus). J Nat Hist 55(5-6):271–282
Winter K, Adams L, Thorp R, Inouye D, Day L, Ascher J, Buchmann S (2006) Importation of non-native bumble bees into North America: potential consequences of using Bombus terrestris and other non-native bumble bees for greenhouse crop pollination in Canada, Mexico, and the United States. North American Pollinator Protection Campaign (NAPPC)
Yoneda M, Furuta H, Tsuchida K, Okabe K, Goka K (2008) Commercial colonies of Bombus terrestris (Hymenoptera: Apidae) are reservoirs of the tracheal mite Locustacarus buchneri (Acari: Podapolipidae). Appl Entomol Zool 43(1):73–76
Yoon HJ, Kim SY, Lee KY, Lee SB, Park IG, Kim I (2009) Interspecific hybridization of the bumblebees Bombus ignitus and B. terrestris. Int J Indust Entomol 18(1):41–48
Acknowledgements
The authors wish to thank the Tuscan Archipelago National Park for its collaboration in this study. We also thanks Mariella Centurioni, Alessandro Palladino, Monica Nardini, Roberta Bonomo, Rossana Chierichetti and Massimo Schiavelli for allowing us the possibility to perform the sampling.
Funding
Open access funding provided by Università di Pisa within the CRUI-CARE Agreement. This research was funded by Tuscan Archipelago National Park, project name “Studio sugli insetti impollinatori e sull’agricoltura tradizionale presso le isole di Capraia e del Giglio” within the Direttiva Biodiversità 2019-2020 of Italian Ministry of Ecological Transition.
Author information
Authors and Affiliations
Contributions
Conceptualization: AF, CBB, FC. Field work: CBB, FC. Analysed the data: AF, CBB, FC, MQ, FG. Taxonomic identification: MQ. Wrote the original draft of the manuscript: AF, CBB, FC. Review and editing the final version of the manuscript: AF, CBB, FC, MQ, FG. Supervision: AF
Corresponding author
Ethics declarations
Institutional review board
Not applicable
Conflict of interest
The authors declare no conflict of interest. One of the authors, Francesca Giannini, is a biologist of the Tuscan Archipelago National Park; we planned and written the paper together.
Additional information
Communicated by: Matthias Waltert
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boni, C.B., Coppola, F., Quaranta, M. et al. Bombus terrestris terrestris (Linnaeus, 1758) and hybrids with the endemic Bombus xanthopus spotted on Capraia Island (Tuscan Archipelago, Italy): some conservation management implications. Sci Nat 110, 14 (2023). https://doi.org/10.1007/s00114-023-01843-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00114-023-01843-y