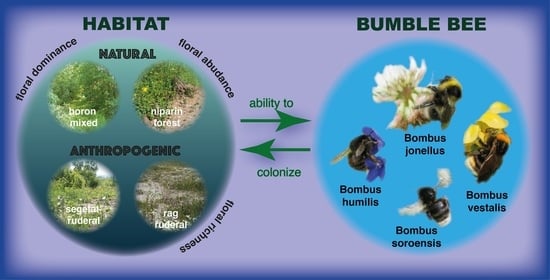
Effects of Open and Forest Habitats on Distribution and Diversity of Bumblebees (Bombus) in the Małopolska Upland (Southern Poland): Case Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site Description
2.2. Collections
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Streinzer, M.; Chakravorty, J.; Neumayer, J.; Megu, K.; Narah, J.; Schmitt, T.; Bharti, H.; Spaethe, J.; Brockmann, A. Species composition and elevational distribution of bumble bees (Hymenoptera, Apidae, Bombus Latreille) in the East Himalaya, Arunachal Pradesh, India. ZooKeys 2019, 851, 71–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ollerton, J.; Winfree, R.; Tarrant, S. How many flowering plants are pollinated by animals? Oikos 2011, 120, 321–326. [Google Scholar] [CrossRef]
- Fitzpatrick, U.; Murray, T.E.; Paxton, R.J.; Breen, J.; Cotton, D.; Santorum, V.; Brown, M.J.F. Rarity and decline in bumblebees-a test of causes and correlates in the Irish fauna. Biol. Conserv. 2007, 136, 185–194. [Google Scholar] [CrossRef]
- Sikora, A.; Michołap, P.; Kadej, M.; Sikora, M.; Tarnawski, D.P. Bees in the City. Bumblebees of Wroclaw; Copyright Stowarzyszenie Natura i Człowiek: Wrocław, Poland, 2018; pp. 1–321. [Google Scholar]
- Bommarco, R.; Lundin, O.; Smith, H.G.; Rundlof, M. Drastic historic shifts in bumblebee community composition in Sweden. Biol. Sci. Proc. R. Soc. Lond. 2012, 279, 309–315. [Google Scholar]
- Goulson, D.; Hanley, M.E.; Darvill, B.; Ellis, J.; Knight, M.E. Causes of rarity in bumblebees. Biol. Conserv. 2005, 122, 1–8. [Google Scholar] [CrossRef]
- Lye, C.L.; Osnorne, J.L.; Park, K.J.; Goulson, D. Using citizen science to monitor Bombus populations in the UK: Nesting ecology and relative abundance in the urban environment. J. Insect Conserv. 2012, 16, 697–707. [Google Scholar] [CrossRef]
- Rasmont, P.; Mersch, P. First estimation of faunistic drift by bumblebees of Belgium, (Hymenoptera: Apidae). Ann. Soc. R. Zool. Belg. 1988, 118, 141–147. [Google Scholar]
- Goulson, D. Bumblebees, Behavior, Ecology and Conservation, 2nd ed.; Oxford University Press: Oxford, UK, 2010; pp. 1–317. [Google Scholar]
- Goulson, D.; Lye, G.C.; Darvill, B. Decline and conservation of bumble bees. Annu. Rev. Entomol. 2008, 54, 191–208. [Google Scholar] [CrossRef]
- Gallai, N.; Salles, J.; Settele, J.; Vaissiere, B.E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009, 68, 810–821. [Google Scholar] [CrossRef]
- Leonhardt, S.D.; Gallai, N.; Garibaldi, L.A.; Kuhlmann, M.; Klein, A.M. Economic gain, stability of pollination and bee diversity decrease from southern to northern Europe. Basic Appl. Ecol. 2013, 14, 461–471. [Google Scholar] [CrossRef]
- Hines, H.; Hendrix, S.D. Bumble bee (Hymenoptera: Apidae) diversity and abundance in tallgrass prairie patches: Effects of local and landscape floral resources. Environ. Entomol. 2015, 34, 1477–1484. [Google Scholar] [CrossRef]
- Geslin, B.; Gauzens, B.; Thebault, E.; Dajoz, I.; Ollerton, J. Plant pollinator networks along a gradient of urbanization. PLoS ONE 2013, 8, e63421. [Google Scholar] [CrossRef]
- Martins, A.C.; Goncalves, R.B.; Melo, G.A.R. Changes in wild bee fauna of a grassland in Brazil reveal negative effects associated with growing urbanization during the last 40 years. Zoologia 2013, 30, 157–176. [Google Scholar] [CrossRef]
- Williams, N.N.; Winfree, R. Local habitat characteristics but not landscape urbanization drive pollinator visitation and native plant pollination in forest remnants. Biol. Conserv. 2013, 160, 10–18. [Google Scholar] [CrossRef]
- Dehon, M.; Engel, M.S.; Gerard, M.; Aytekin, M.; Ghisbain, G.; Williams, P.H.; Rasmont, P.; Michez, D. Morphometric analysis of fossil bumble bees (Hymenoptera, Apidae, Bombini) reveals their taxonomic affinities. ZooKeys 2019, 891, 71–118. [Google Scholar] [CrossRef] [Green Version]
- Riis, T.; Kelly-Quinn, M.; Aguiar, F.C.; Manolaki, P.; Bruno, D.; Bejarano, M.D.; Clerici, N.; Fernandes, M.R.; Franco, J.C.; Pettit, N. Global Overview of Ecosystem Services Provided by Riparian Vegetation. BioScience 2020, 70, 501–514. [Google Scholar] [CrossRef]
- Ruszkowski, A.; Biliński, A.; Kosior, A.; Bąk, J.; Kaczmarska, K. The bumble bees of Małopolska Upland. Pszczel. Zesz. Naukowe 1989, 3, 33–44. [Google Scholar]
- Bąk, J. Preliminary research on bumblebees (Bombus Latr.) and cuckoobees (Psithyrus Lep.) (Hymenoptera: Bombini) of the Polana Polichno steppe reserve in the Świętokrzyskie Province. Wiad. Entomol. 2006, 25, 21–27. [Google Scholar]
- Matuszkiewicz, W. Przewodnik do Oznaczania Zbiorowisk Roślinnych Polski; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2021; pp. 1–210. [Google Scholar]
- Mirek, Z.; Piękoś-Mirkowa, H.; Zając, A.; Zając, M. Flowering Plants and Pteridiophytes of Poland. A Checklist; W. Szafer Institute of Botany, PAN: Krakow, Poland, 2002; pp. 1–441. [Google Scholar]
- Szafer, W. Szata Roślinna Polski Niżowej; Wydawnictwo Naukowe PWN: Warszawa, Poland, 1977; pp. 1–638. [Google Scholar]
- Krzysztofiak, A.; Krzysztofiak, L.; Pawlikowski, T. Trzmiele Polski—Przewodnik Terenowy; Wydawnictwo UMK: Toruń, Poland, 2004; pp. 1–48. Available online: https://backend.pomagamypszczolom.pl/media/attachments/Trzmiele-Polski-Przewodnik-Terenowy.pdf (accessed on 7 December 2019).
- Pawlikowski, T. A Distribution atlas of Bumblebees in Poland; Nicolaus Copernicus University Press: Torun, Poland, 2008; pp. 1–104. [Google Scholar]
- Dylewska, M.; Flaga, S. Barwny Klucz Do Rozpoznawania w Warunkach Polowych Krajowych Gatunków Trzmieli; Wyd. Polski Klub Ekologiczny: Warszawa, Poland, 2000; pp. 1–80. [Google Scholar]
- Dylewska, M.; Gąsienica-Chmiel, M.; Kosior, A.; Sumera, A.; Szafraniec, S.; Werstak, K.; Wiśniowski, B. Skład gatunkowy i liczebność trzmieli i trzmielców (Bombinae, Apoidea, Hymenoptera) na łąkach w wybranych parkach narodowych oraz kwiecistość łąk w parkach w 1998 roku. Prądnik 1998, 11, 279–292. [Google Scholar]
- Williams, P.H.; Osborne, J.L. Bumblebee vulnerability and conservation world-wide. Apodologie 2009, 40, 367–387. [Google Scholar] [CrossRef] [Green Version]
- Banaszak, J. Bumble Bees of Poland; Wydawnictwo WSP Bydgoszcz: Bydgoszcz, Poland, 1993; pp. 1–158. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Paleontological Statistic software package for education and data analysis. Paleontol. Elektron. 2001, 4, 1–9. [Google Scholar]
- StatSoft. Inc. STATISTICA (data analysis software system), version 9.0. 2009. Available online: www.statsoft.com (accessed on 7 December 2019).
- Ter Braak, C.J.F.; Smilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination; Version 5.0; Microcomputer Power: Ithaca, NY, USA, 2012; pp. 1–496. [Google Scholar]
- Westphal, C.; Steffan-Dewenter, I.; Tscharntke, T. Mass flowering crops enhance pollinator densities at a landscape scale. Ecol. Lett. 2003, 6, 961–965. [Google Scholar] [CrossRef]
- Person, A.S.; Rundlof, M.; Clough, Y.; Smith, H.G. Bumble bees show trait-dependent vulnerability to landscape simplication. Biodivers. Conserv. 2015, 24, 3469–3489. [Google Scholar] [CrossRef]
- Krzysztofiak, A. Struktura zgrupowań pszczół (Apoidea, Hymenoptera) w różnowiekowych drzewostanach świerkowo-sosnowych Wigierskiego Parku Narodowego. Zeszyty Naukowe Akademii Bydgoskiej Studia Przyrodnicze 2001, 15, 113–215. [Google Scholar]
- Pawlikowski, T. Struktura Zespołów Pszczołowatych (Hymenoptera, Apoidea) na Obszarach Leśnych Kotliny Toruńskiej; Wydawnictwo Naukowe UMK Press: Torun, Poland, 1992; pp. 1–153. [Google Scholar]
- Gómez-Martinez, C.; Aase, A.L.T.; Totland, Q.; Rodriguez-Pérez, J.; Birkemoe, T.; Sverdrup-Thygeson, A.; Lázaro, A. Forest fragmentation modifies the composition of bumblebee communities and modulates their trophic and competitive interactions for pollinantion. Sci. Rep. 2020, 10, 10872. [Google Scholar] [CrossRef]
- Nieto, A.; Roberts, S.; Kemp, J.; Rasmont, P.; Kuhlmann, M.; Criado, M.G.; Biesmeijer, J.C.; Bogusch, P.; Dathe, H.H.; de la Rúa, P.; et al. European Red List of Bees; Publication Office of the European Union: Luxembourg, 2014; pp. 1–98. [Google Scholar]
- Osborne, J.L.; Williams, L.H. Bumble bees as pollinators of crops and wild flowers. In Bumble Bees for Pleasure and Profit International Bee Research Association; Matheson, A., Ed.; International Bee Research Association: Cardiff, Wales, 1996; pp. 24–32. [Google Scholar]
- Głowaciński, Z.; Nowacki, J. Polska czerwona księga zwierząt. In Bezkręgowce; IOP PAN: Krakow, Poland, 2004; pp. 1–447. [Google Scholar]
- Sarospataki, M.; Novak, J.; Molnar, V. Assessing the threatened status of bumble bee species (Hymenoptera: Apidae) in Hungary, Central Europe. Biodivers. Conserv. 2005, 14, 2437–2446. [Google Scholar] [CrossRef]
- Rollin, O.; Vray, S.; Dendoncker, N.; Michez, D.; Dufrêne, M.; Rasmont, P. Drastic shifts in the Belgian bumblebee community over the last century. Biodivers. Conserv. 2020, 29, 2553–2573. [Google Scholar] [CrossRef]
- Ornosa, C. Bombus confuses (Schenck, 1861). In Atlas y Libro Rojo de Los Invertebrados Amenazados de Espana (Espesies Vulnerables); Verdů, J.R., Numa, C., Galante, F., Eds.; Direction General de Medio Natural y Politica Forestal, Ministerio de Medio Ambiente Medio rural y: Marino, Spain, 2011; pp. 1174–1178. [Google Scholar]
- Potapov, G.S.; Kolosova, Y. Bombus (Pyrobombus) jonellus (Kirby, 1802) in the north-western Russian Plain: Its distribution and ecology. Arct. Environ. Res. 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Delmas, R. Contribution a l’etude de la faune francaise des Bombidae (Hymenoptera, Apoidea, Bombidae). Ann. Soc. Entomol. Fr. 1976, 12, 247–290. [Google Scholar]
- Morales, C.; Arbetman, M.P.; Cameron, S.A.; Aizen, M.A. Rapid ecological replacement of native bumble bee by invasive species. Front. Ecol. Environ. 2013, 11, 529–534. [Google Scholar] [CrossRef]
- Tehel, A.; Brown, M.J.F.; Paxton, R.J. Impact of managed honey bee viruses on wild bees. Curr. Opin. Virol. 2016, 19, 16–22. [Google Scholar] [CrossRef] [Green Version]
- Black, S.H.; Shepard, M.; Allen, M.M. Endangered invertebrates: The case for greater attention to invertebrate conservation. Endanger. Species UPDATE 2001, 18, 42–50. [Google Scholar]
- Carvell, C. Habitat use and conservation of bumblebees (Bombus spp.) under different grassland management regimes. Biol. Conserv. 2002, 103, 33–49. [Google Scholar] [CrossRef]
- Michołap, P.; Sikora, A.; Pawlikowski, T.E.; Sikora, M. Dispersion of Bumblebee Bombus Semenoviellus Skorikov (Hymenoptera, Apidae) in Poland. J. Apic. Sci. 2020, 64, 47–54. [Google Scholar] [CrossRef]
- Scoble, J. The Lepidoptera: Form, Function and Diversity; Oxford University Press: New York, NY, USA, 1992; pp. 1–404. [Google Scholar]
- Michener, D.D. The Bees of the World, 2nd ed.; Johns, M.D., Ed.; Hopkins University Press: Baltimore, MD, USA, 2007; pp. 1–953. [Google Scholar]
- Winfree, R.; Bartomeus, I.; Cariveau, D.P. Native pollinators in anthropogenic habitats. Annu. Rev. Ecol. Syst. 2011, 42, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Steffan-Dewenter, I.; Munzenberg, U.; Burger, C.; Thies, C.; Tscharntke, T. Scale dependent effects of landscape context on three pollinator guilds. Ecology 2002, 83, 1421–1432. [Google Scholar] [CrossRef]
- Sjodin, N.E.; Bengtsson, J.; Ekbom, B. The influence of grazing intensity and landscape composition on the diversity and abundance of flower visiting insects. J. Appl. Ecol. 2008, 45, 763–772. [Google Scholar] [CrossRef]
- Williams, P. An annotated checklist of bumble bees with an analysis of patterns of description (Hymenoptera: Apidae, Bombini). Biulletin Nat. Hist. Mus. Entomol. 1998, 67, 79–152. [Google Scholar]
- Tews, J.; Brose, U.; Grimm, V.; Tielborger, K.; Wichmann, M.C.; Schwager, M.; Jeltsch, F. Animal species diversity driven by habitat heterogeneity/diversity: The importance of keystone structures. J. Biogeogr. 2004, 31, 79–92. [Google Scholar] [CrossRef] [Green Version]
- Klein, A.M.; Steffan-Dewenter, I.; Tscharntke, T. Fruit set of highland coffee increases with the diversity of pollinating bees. Proc. R. Soc. Lond. Ser. B 2003, 270, 955–961. [Google Scholar] [CrossRef] [Green Version]
- Zajdel, B.; Boranski, M.; Kucharska, K.; Jojczyk, A.; Brzezinska, K. Bumblebee communities (Apidae, Bombini) in urban parks in relations to park area and other characteristics. Pol. J. Ecol. 2019, 67, 84–93. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Forero, I.; Kuusemets, V.; Mand, M.; Liivamagi, A.; Kaart, T.; Luig, J. Effects of forest habitats on the local abundance of bumblebee species: A landscape-scale study. Balt. For. 2011, 17, 235–242. [Google Scholar]
- Memmott, J.; Craze, P.G.; Waser, N.M.; Price, M.V. Global warming and the disruption of plant-pollinator interactions. Ecol. Lett. 2007, 10, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Kleijn, D.; Rundlöf, M.; Scheper, J.; Smith, H.G.; Tscharntke, T. Does conservation on farmland contribute to halting the biodiversity decline? Trends Ecol. Evol. 2011, 26, 474–481. [Google Scholar] [CrossRef] [PubMed]









| SNP | COLP | ChKLP | NLP | |||||
|---|---|---|---|---|---|---|---|---|
| Species | Specimens | Species | Specimens | Species | Specimens | Species | Specimens | |
| 2003 | 13 | 76 | 15 | 257 | 15 | 117 | 15 | 93 |
| 2004 | 11 | 91 | 19 | 183 | 12 | 150 | 14 | 150 |
| 2005 | 15 | 152 | 17 | 516 | 15 | 257 | 16 | 183 |
| 2006 | 15 | 179 | 17 | 414 | 17 | 532 | 18 | 321 |
| 2017 | 11 | 44 | 14 | 164 | 14 | 95 | 15 | 66 |
| 2018 | 10 | 60 | 16 | 150 | 12 | 114 | 18 | 110 |
| 2019 | 14 | 97 | 15 | 365 | 15 | 193 | 16 | 106 |
| 2020 | 14 | 127 | 14 | 305 | 15 | 321 | 17 | 226 |
| 2003/2004 | 2005/2006 | 2017/2018 | 2019/2020 | ||
|---|---|---|---|---|---|
| n | COLP | 48/59 | 138/167 | 123/141 | 112/118 |
| sn | 69/91 | 119/365 | 105/225 | 253/187 | |
| n | NLP | 23/48 | 65/166 | 54/152 | 36/114 |
| sn | 70/102 | 118/155 | 102/136 | 73/112 | |
| n | ChKLP | 168/123 | 252/284 | 240/380 | 202/214 |
| sn | 89/60 | 264/130 | 200/125 | 185/105 | |
| n | SNP | 16/29 | 66/81 | 54/88 | 41/52 |
| sn | 60/62 | 86/98 | 76/78 | 56/75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bąk-Badowska, J.; Wojciechowska, A.; Czerwik-Marcinkowska, J. Effects of Open and Forest Habitats on Distribution and Diversity of Bumblebees (Bombus) in the Małopolska Upland (Southern Poland): Case Study. Biology 2021, 10, 1266. https://doi.org/10.3390/biology10121266
Bąk-Badowska J, Wojciechowska A, Czerwik-Marcinkowska J. Effects of Open and Forest Habitats on Distribution and Diversity of Bumblebees (Bombus) in the Małopolska Upland (Southern Poland): Case Study. Biology. 2021; 10(12):1266. https://doi.org/10.3390/biology10121266
Chicago/Turabian StyleBąk-Badowska, Jolanta, Anna Wojciechowska, and Joanna Czerwik-Marcinkowska. 2021. "Effects of Open and Forest Habitats on Distribution and Diversity of Bumblebees (Bombus) in the Małopolska Upland (Southern Poland): Case Study" Biology 10, no. 12: 1266. https://doi.org/10.3390/biology10121266